Technology Information Sheet
Ion Exchange
June 2004 Fact Sheet 2004-05
What are Ion Exchange Resins?
Ion exchange resins can be made from synthetic or natural materials shaped into grain-of-sand-sized beads.
Ions are negatively or positively charged atoms or molecules. The ion exchange resins have the opposite charge of the contaminant ions they are treating. This causes contaminants in water pumped through a container of ion exchange resin to be attracted to and stick to the resin similar to the way metal reacts to a magnet. When the contaminant ion attaches to the resin, it displaces another similarly charged ion into the water being treated. That ion is "exchanged" for the contaminant.

Ion Exchange Resin Beds (Courtesy of the Purolite Company)
Home water-softener systems use ion exchange to draw out the chemicals that make the water hard. The exchange ion in these systems is usually sodium.
At Camp Edwards, ion exchange resins will be used to remove perchlorate, a negatively charged ion, from groundwater. The "exchange" ion will be chloride - an ingredient in table salt that is naturally occurring in Cape Cod groundwater.
/
When perchlorate fills up the available space on the ion exchange resin, the resin will be replaced. Used resin will be disposed of according to environmental regulations.
Proven Process
Ion Exchange Resins are approved for treating drinking water in California, Washington and Colorado.
The resins have been used to treat perchlorate contaminated groundwater at sites including the Municipal Water Districts in La Puente and San Martin, Calif.; Edwards Air Force Base, Calif., and at the Kerr-McGee facility near Las Vegas, Nev. Results from sampling the treated groundwater at these sites showed all contaminants were eliminated to below the detection level for the approved EPA laboratory method (4 parts per billion).

Camp Edwards Ion Exchange Test Container
Field-Scale Study at Camp Edwards
Since other sites using ion exchange resins to treat perchlorate use a detection level of 4 parts per billion (ppb), the Impact Area Groundwater Study Program commissioned a pilot study to determine the ability of ion exchange resins to remove perchlorate from groundwater to the lower EPA approved detection levels used at Camp Edwards (0.35 ppb).
The field-scale study currently underway is testing two types of ion exchange resins to determine their ability to treat perchlorate to levels below the 0.35 detection limit.
Type I Styrene Resin has been shown to successfully remove perchlorate to the 4 ppb level at other sites and is considered a reliable ion exchange resin. Nitrate Selective Resins are resins designed to specifically attract perchlorate-like ions, and provides up to 4 times the removal efficiencies of Type I Styrene Resin.
The Camp Edwards pilot study began in January 2004, and will be completed in summer 2004. Results from this evaluation will help determine the most effective and efficient way to remove perchlorate from groundwater at Camp Edwards.
How It Works
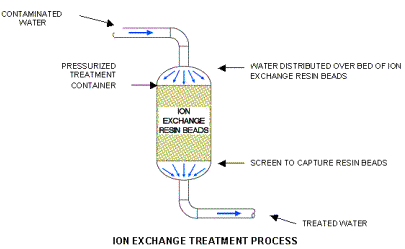
Ion Exchange Resins removes perchlorate by using a positively charged resin to capture negatively charged perchlorate ions from water passing through a container of ion exchange beads and release naturally occurring chloride ions in their place.
A typical ion exchange system consists of an enclosed container filled with millions of small resin beads. The contaminated water is pumped through the container and flows between the beads. The perchlorate ion is attracted to the resin, where it attaches and displaces a chloride ion. The chloride ion replaces the perchlorate ion in the groundwater.
The amount of perchlorate captured by the ion exchange resin depends on:
- the amount of perchlorate in the groundwater;
- the number and level of competing ions, such as nitrates, in the groundwater;
- the size of the resin beads; and
- the amount of time the water spends in the ion exchange container (residence time).
The amount of chloride ions introduced to the groundwater depends on the levels of perchlorate and competing ions, like nitrate in the groundwater being treated. At Camp Edwards, the concentrations of these ions are low, so very little chloride will be added to the groundwater during the ion exchange process.
The treated water passes through a screen small enough to hold back the resin beads before the water exits the container and is discharged back into the aquifer using reinjection wells. Treated water is sampled frequently to confirm the ion exchange resin is effectively removing the perchlorate.
Over time, perchlorate and competing ions will saturate the ion exchange resin, requiring it to be changed for new resin. Due to the low levels of perchlorate being treated at Camp Edwards, the resins are expected to have a long life. Over time, small, fine particles of soil may clog the resin beads, requiring them to be changed. It is projected that at Camp Edwards, this will occur before the resin needs to be changed due to saturation.
Used resin will be treated and/or disposed of in accordance with environmental regulations.
|



